The Covid 19 pandemic has now changed the way most of us are going about our day to day lives. In India, we are now on day 2 of our 21 day nation wide lock-down. In the midst of all that is going on now, Intellectual Property enthusiasts with a keen eye, would have noticed several IP related issues that have been cropping up, even if relatively under-discussed. For this current post, I thought I would do a round-up of various currently relevant IP issues for our readers to ponder upon.
The Covid 19 pandemic has now changed the way most of us are going about our day to day lives. In India, we are now on day 2 of our 21 day nation wide lock-down. In the midst of all that is going on now, Intellectual Property enthusiasts with a keen eye, would have noticed several IP related issues that have been cropping up, even if relatively under-discussed. For this current post, I thought I would do a round-up of various currently relevant IP issues for our readers to ponder upon.
So far on the blog, we’ve already had a few Covid 19 related posts. A couple of weeks ago I wrote on ‘Patent Politics in the time of Corona‘ and more recently Latha wrote about practical concerns in her post ‘COVID-19: How Best Can the Indian IP Office Help its Stakeholders?’. Prashant has also just written a hard hitting post on the urgent need for an IP task force and IP Policy to deal with the mass shortage of masks, medical equipment, etc. He ends with the lines “Parliament in its wisdom foresaw such public health scenarios when it provided for remedies such as compulsory licensing or patent acquisitions. If not now, then when will we use these provisions?”
It’s become amply clear that in the light of people fearing for their safety, IP Flexibilities, Openness, and Access have suddenly become much more significant in people’s minds. While these are principles that the several Public-interest related movements have been fighting for, for decades, it has taken a global pandemic for them to be finally taken seriously. One hopes that once this passes, the memory of these reasons don’t pass with it. In this post, I merely intend to throw up (hopefully provocative) questions that seem relevant and worth examining. As always, we are happy to consider guest posts on these topics, or any topic that may be relevant to the blog. Without further ado, here we go:
Open Access / Open Science:
“Health is perhaps the area of most intense demand for greater access to scientific and technical information, partly because failure to obtain it can be literally fatal”. H. Momen did not pre-empt the current situation when writing this statement in the 2003 WHO Bulletin. It was a reality then, and it is a reality now. Almost as soon as it became clear that Covid 19 was going to be serious, there were several calls for open sharing of information, research and data regarding the virus. Nature put out an editorial in early February, titled, ‘Calling all coronavirus researchers: keep sharing, stay open‘. To several, this was a new way of going about their research (see here). Xin Xu from Univ of Oxford has put out very detailed note on several of the Open Access initiatives with respect to Covid 19, while also discussing ‘responsible’ open science – click here to read it.

Image from here
What if this had remained copyright protected, with life of author plus 60 years as the protected period? Why shouldn’t it have? Should we necessarily rely on the largess of publishers to be charitable in times of need? Does that mean they can decide what is a time of need? Academic publishing in particular is both notoriously expensive, as well as very well understood to not really be giving any financial recompense to the authors. Of course, there are several benefits that big publishers bring to the table. However, it is surely time for a more sustainable and equitable publishing model to be taken up more seriously.
Before moving on to other areas, I’m reproducing a notable example of the impact that copyright can have on public health as given in this paper:
“I recently met a physician from southern Africa, engaged in perinatal HIV prevention, whose primary access to information was abstracts posted on the Internet. Based on a single abstract, they had altered their perinatal HIV prevention program from an effective therapy to one with lesser efficacy. Had they read the full text article they would have undoubtedly realized that the study results were based on short- term follow-up, a small pivotal group, incomplete data, and were unlikely to be applicable to their country situation. Their decision to alter treatment based solely on the abstract’s conclusions may have resulted in increased perinatal HIV transmission.”
– Arthur Amman, President of Global Strategies for HIV Prevention
Access to culture:
However, open access hasn’t just been about access to scientific articles. Several are realising that being quarantined also means that many no longer have access to recreation, culture and socialising activities – the lack of which is a disincentive for staying indoors. Audible, for instance, has opened up its younger audience focused audiobooks for as long as schools remain closed. Closer home, Amar Chitra Katha Media is offering a free one month subscription to everyone, till the end of March. Moves such as these, are of course, more likely brand building and sales related tactics more than addressing ‘access’ related concerns. Nonetheless, it does make one ponder over the fact that access to culture (if I can use that term here) is important enough that publishing houses realise that in times when access is denied, providing such access helps build their brand image too. Perhaps worth considering the importance of libraries vis-a-vis access to culture, in not so disastrous times, for those who cannot afford to purchase it otherwise. On the note of libraries, the Internet Archive has also just announced a National Emergency Library (for USA) to deal with the emergent wait list problem that is now being faced by the nation’s ‘displaced learners’. Click here to read about it. That term ‘displaced learners’ is quite a useful one I would think – since it emphasises that not all ‘students’ may be in schools, or even be able to be in schools – reemphasizing the importance of having learning materials available to the public.
Speaking of access to culture – it would be extremely interesting to know whether Netflix (who along with YouTube have been asked to reduce their streaming quality to prevent the Internet from collapsing!) , Amazon Prime, etc have seen a boost in subscriptions over the last couple of weeks. Steam, the popular gaming platform, has already reported its highest ever numbers. For that matter, what changes in ‘piracy’ rates have taken place? Based on the content from a number of WhatsApp forwards I’ve received with a title along the lines of “Quarantine starter pack”, my guess is that it has also risen dramatically – but it would be interesting to see whether it has increased in proportion with the rise in ‘legal’ streaming services, or more, or less. Then there are other questions as well. In these times of unprecedented ‘social distancing’ – how does the rise of digital based access to culture affect persons with visual disabilities or other print disabilities? Yes, there are already certain initiatives such as Sugamya Pustakalaya. However, what about initiatives specific to the burdens of social distancing? In other words – while students from the average middle class family who are now sitting at home can use online gaming or instagramming or whatever else for their ‘socialising’ and ways of passing time, are there accessible methods available to persons with disabilities? Are there copyright exceptions and flexibilities that are being underused / still not present? If ever there was a time to examine those questions – it would be now.
Patent ownership, Compulsory Licensing and Clinical Trial Data
(Several links in this section are from the discussions over at KEI’s IP-Health listserv)
I’ve already touched upon several of Big Pharma issues in my earlier post, and Prashant’s post touched upon issues that the Government would be wise to look into for immediate measures during this pandemic. However a lot more has been happening as well. For the sake of brevity, I’ll mention these just in the form of updates.
As I had earlier discussed, Remdesivir is one of the treatments that is appearing promising. It had started as a treatment for ebola, but clinical trials showed that it turned out to be not as effective as first thought. From what I can tell from this link, Remdesivir was developed by the University of Alabama at Birmingham (UAB), and Gilead Sciences in a Private Public Partnership. I am guessing (and open to correction) that this means the initial research and development was done by UAB and it was taken to clinical trials by Gilead. Coming back to present times – China’s Wuhan Institute of Virology, which seems to have first discovered the potential of Remdesivir as a treatment for Covid 19, appears to have beat Gilead to the punch and filed alternate medical use patents around the world. Even if granted, it seems these may be dependent on Gilead’s earlier patents. Meanwhile on the side lines, there is another interesting development unfolding as BrightGene, a Chinese biotech company seems to be wondering whether they can launch a generic version of remdesivir without approval of the patent holders – since they plan on ‘donating’ the drug to patients. See more on that here.
There are of course other potential treatment drugs – such as Kaletra. Kaletra is Abbvie’s brand name for a fixed dose combination of lopinavir / ritonavir, which is used in the treatment of HIV / AIDS. As it turns out, the treatment is being considered favourably in Israel for treating Covid 19 (though there is a paper that says otherwise). Due to Abbvie’s inability to supply enough of the medicine, Israel invoked a compulsory license – for Indian generic Hetero to import their generic version to Israel, for the purpose of Covid-19 treatment. See more on this here. It’s worth noting that the fear of not having sufficient supplies of a drug that might not even work was sufficient for Israel to issue a Compulsory Licence. (One wonders if Israel will come under the same kind of 8-years-and-running-criticism that India’s been receiving for its CL usage back in 2012, for a drug that actually did work). As per this update, the generic version is available in India because the Indian Patent Office rejected Abbvie’s patent application earlier. A few days after Israel issued this compulsory licence, Abbvie declared it would not enforce these patents globally.
Aside from Israel though, other countries too have made loud and clear signals that Compulsory Licensing options are definitely on the table. As KEI reports, Chilean Chamber of Deputies approves resolution for compulsory licenses for patents relating to the coronavirus virus and Legislative Committee in Ecuador approves resolution on compulsory licensing of patents relating to the coronavirus. The Kluwer Patent Blog notes that Germany is also considering similar action, and it appears Canada is also preparing for more CL type activity (see their Patent Act amendment here). Will the first world pressure against the use of Compulsory Licences continue, even after this pandemic has proven how necessary they are? Would it even be in their interests to start actively advocating for more patent flexibilities as a norm? Check here for the MedicinesLawPolicy blog’s post on Covid-19 and the comeback of compulsory licensing
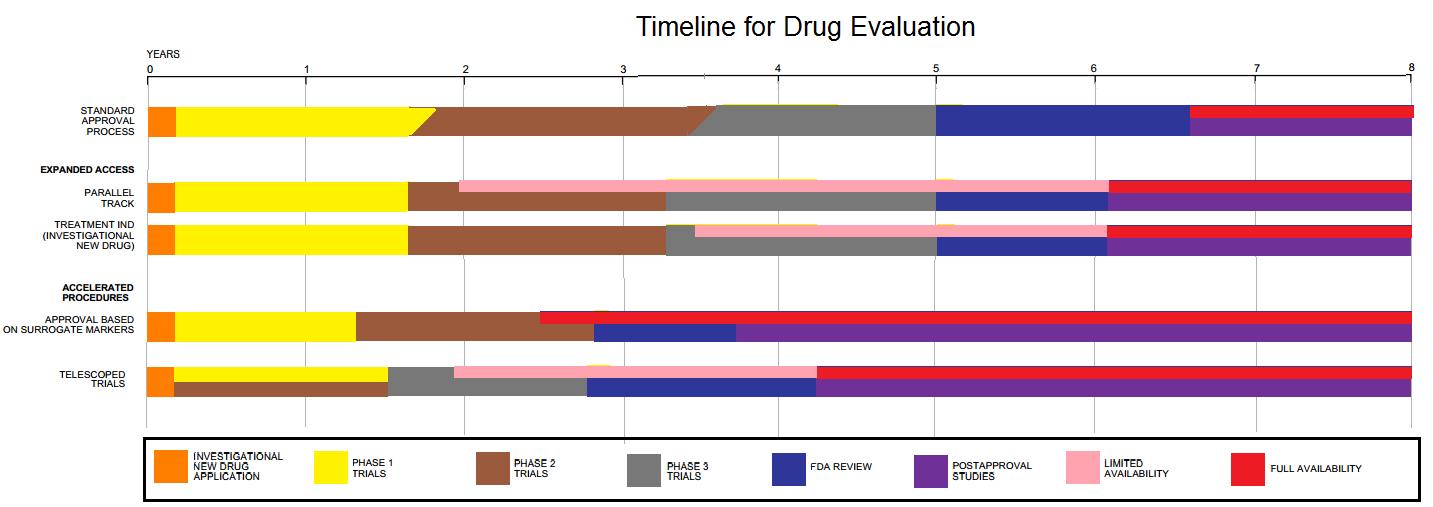
Image from here. Image put in for generalised information on the timelines for drug approvals and research
And even if CLs are no longer a bad word – what about the barriers posed by regulatory exclusivities? See here for eg. Various ‘developed’ pharma patent system allow for exclusivity over clinical trial data – separate from exclusivity granted via patents. This allows owners of such data to prevent generic drug makers from relying on that data for their regulatory approval, thus necessitating separate clinical trials (i.e., many years and a lot of money) by the generic entrant.
Even as Gilead keeps mum about the patent dispute over Remdesivir, it had applied for and received from the US FDA permission to designate Remdesivir as an ‘orphan drug’. Among other things, this designation, (that is reserved for treatments for ‘rare diseases’ i.e.,affecting a tiny minority of the population), allows 7 years of data exclusivity. This article has more, including a mention of Gilead’s former lobbyist being part of the White House’s Corona task force. And here, KEI notes that the FDA tried to keep Gilead’s date of application as confidential, since it would reveal whether they had applied for this very early on when there were very few patients, or whether they had applied much later, after it had become a pandemic. It turns out, they had applied in early March, well after it was far far from a ‘rare disease’, begging the question of why it was granted this status. Soon enough, presumably due to bad press, Gilead requested that this status be rescinded. These developments, while US specific, could have ramifications for other parts of the world as well. As we’ve seen in the past – Big Pharma has been happy to prevent poorer countries from having accessible medicines so long as they have a ‘richer’ market elsewhere. What implications do US’ Public-interest-unfriendly IP policies and captured regulators have on other countries, in times of emergency like now?
Drug Development Models
In addition to the above two treatment possibilities, there are at least two other treatment options that are being seen to have positive potential. In order to quicken things up, the World Health Organisation is organising a global coordinated mega-trial, called SOLIDARITY, for these four treatment options. ScienceMag has a detailed description of it here. With similar motivations of quicker and cheaper treatment – Costa Rica has asked the WHO to help create a global IP pool for technologies relating to Covid 19. As per KEI,:Costa Rica proposes the pool “should include existing and future rights in patented inventions and designs, as well as rights in regulatory test data, know- how, cell lines, copyrights and blueprints for manufacturing diagnostic tests, devices, drugs, or vaccines. It should provide for free access or licensing on reasonable and affordable terms, in every member country.” Is it possible that with this pandemic, R&D – price delinkage will finally start to gain momentum?
More from you?
This post has rambled on for long enough by now. There is a lot more on Covid 19 centric IP issues of course – from what seems to be India’s first Covid19 related IP dispute (case between HUL and Dettol, that SpicyIP will be writing on soon), to various trademark related queries – Would a “Corona Handwash” trademark be valid or is it too descriptive? What about “Karo na handwash”? What is Corona Beer’s take on all of this? What are the IP issues with the various counterfeit issues that are bound to be taking place? I’ll leave you to ponder over these, and perhaps write in to us with some of your thoughts as well. Finally, I’ll leave you with what has been my favorite Covid-IP headline of these last few months – from TechDirt: SoftBank Owned Patent Troll, Using Monkey Selfie Law Firm, Sues To Block Covid-19 Testing, Using Theranos Patents.